|
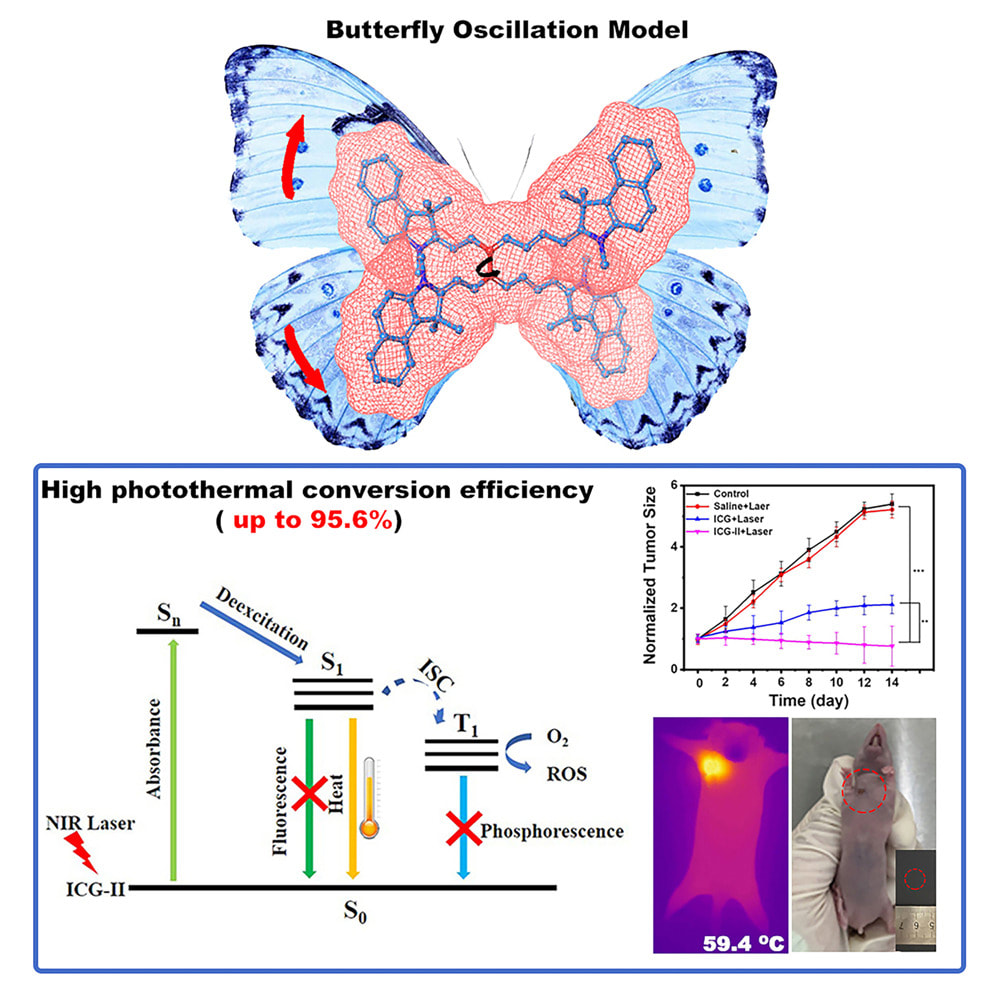
I'm excited to share our latest research published in Cell Reports Physical Science on a game-changing advancement in photothermal therapy for cancer treatment. Our team discovered that the dimer of indocyanine green (ICG), ICG-II, can completely eliminate tumors in just 2 minutes, compared to regular ICG which doesn't work even after 5 minutes.
The key breakthrough centers around what we call "butterfly oscillation." ICG-II is essentially two ICG molecules linked together, and this pairing creates a constrained molecular motion that dramatically improves how efficiently light energy converts to heat. While regular ICG only converts 47% of laser energy to heat, ICG-II achieves an incredible 95.6% conversion efficiency - the highest ever reported for a small-molecule photothermal agent. Here's what makes this so exciting: the molecular structure of ICG-II forces it to undergo a "butterfly" vibration where parts of the molecule flap toward and away from each other. This motion facilitates non-radiative energy release as heat rather than fluorescence, making it vastly more effective at destroying cancer cells through targeted heating. In our mouse studies, direct injection of ICG-II followed by just 2 minutes of near-infrared light completely eliminated xenograft tumors in some cases, with tumor temperatures reaching over 60°C. The treatment was so effective that two mice experienced complete remission. Meanwhile, equivalent doses of regular ICG couldn't achieve tumor clearance even with longer treatment times. What's particularly promising is that ICG-II maintains excellent biocompatibility and is actually a known, FDA-permitted compound that forms naturally as an impurity during ICG production. This could significantly accelerate the path toward clinical applications, potentially offering cancer patients a faster, more effective photothermal treatment option.
0 Comments
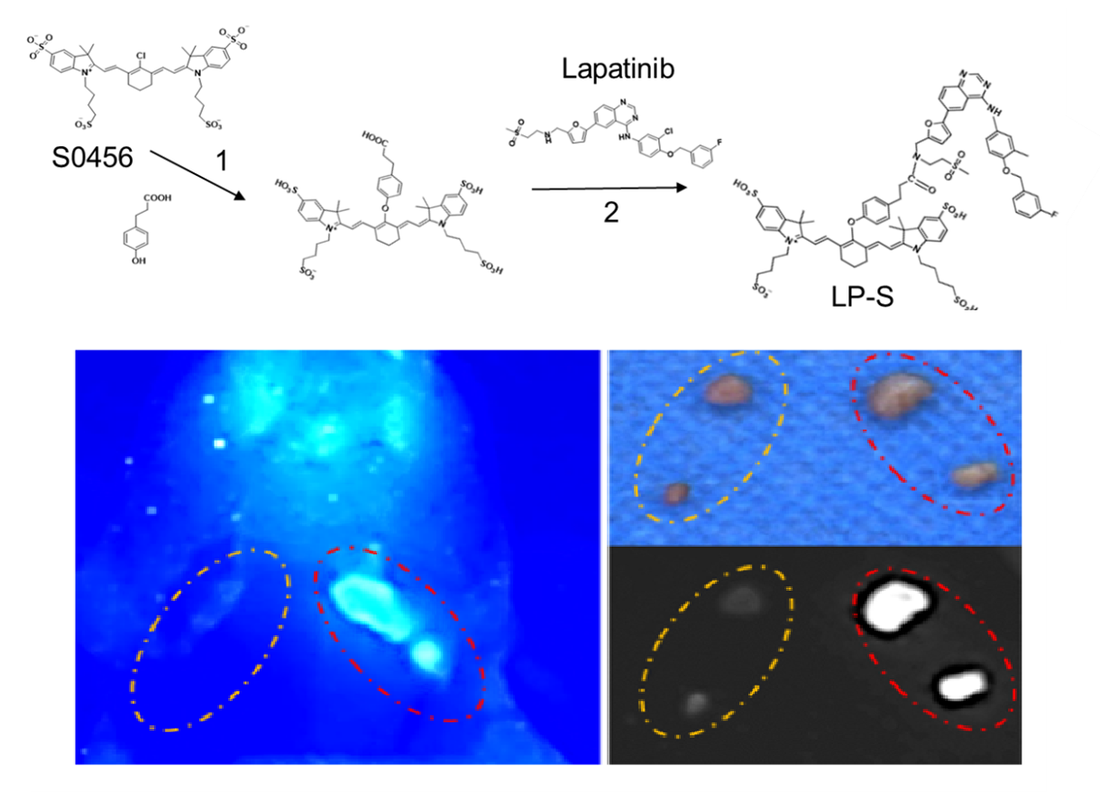
We've developed a promising new tool that could make oral cancer surgery more precise. Our team created a specialized fluorescent dye called LP-S that lights up metastatic lymph nodes, making them easier for surgeons to identify and remove.
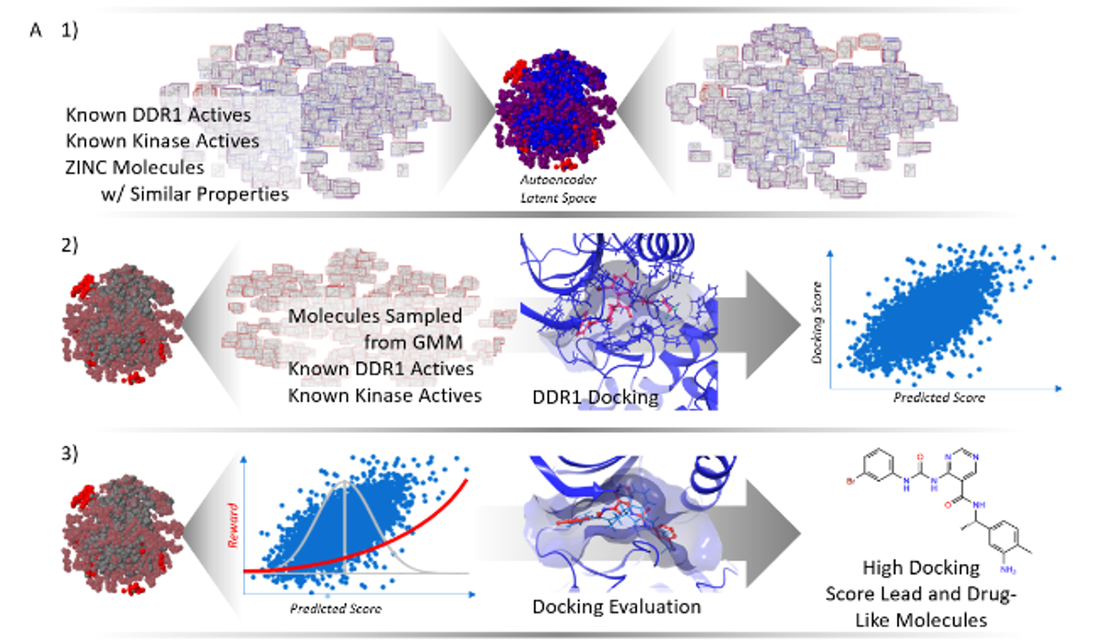
Right now, surgeons rely on a standard dye called ICG, but it's not very specific - it highlights everything, making it hard to tell which lymph nodes actually contain cancer cells. Our new dye is much more precise, specifically binding to EGFR receptors, which are found in high concentrations on oral cancer cells. When we tested LP-S in mouse models, the results were impressive. The dye produced clearer, longer-lasting fluorescence in cancerous lymph nodes compared to ICG, giving surgeons a much better view of what they're working with. This means they can more accurately remove the correct lymph nodes while leaving healthy tissue in place. If this technology makes it through clinical testing, it could be a game-changer for oral cancer patients. Better lymph node mapping means more accurate cancer staging and ultimately better treatment outcomes. Bayesian regression model combining docking and deep generative networks published in JCAMD8/8/2023 We've published new research in the Journal of Computer-Aided Molecular Design that could speed up the hunt for new medicines. Our team developed a hybrid AI model that's better at designing potential drug molecules than traditional computational methods.
Here's how it works: we combined two powerful AI approaches - one that generates new molecular structures and another that predicts how well those molecules will bind to their target protein. By linking these together with reinforcement learning, our system can efficiently explore vast chemical possibilities and focus on the most promising candidates. When we tested this approach on DDR1 kinase (a protein involved in cancer), the results were impressive. Our hybrid model found molecules with much higher binding scores than conventional similarity-based methods. Even better, it discovered chemically diverse compounds that looked quite different from known inhibitors but still bound effectively to the target. What makes this especially exciting is that our approach reduces one of the biggest limitations in AI drug discovery - the need for massive datasets. Instead of just making slight tweaks to existing drugs, our system can venture into unexplored chemical territory to find genuinely novel compounds. While there's still more work ahead, this hybrid strategy could help researchers identify promising new treatments for diseases where current drugs aren't working well enough. |
Author
Chris Archives
June 2025
Categories |
 RSS Feed
RSS Feed
