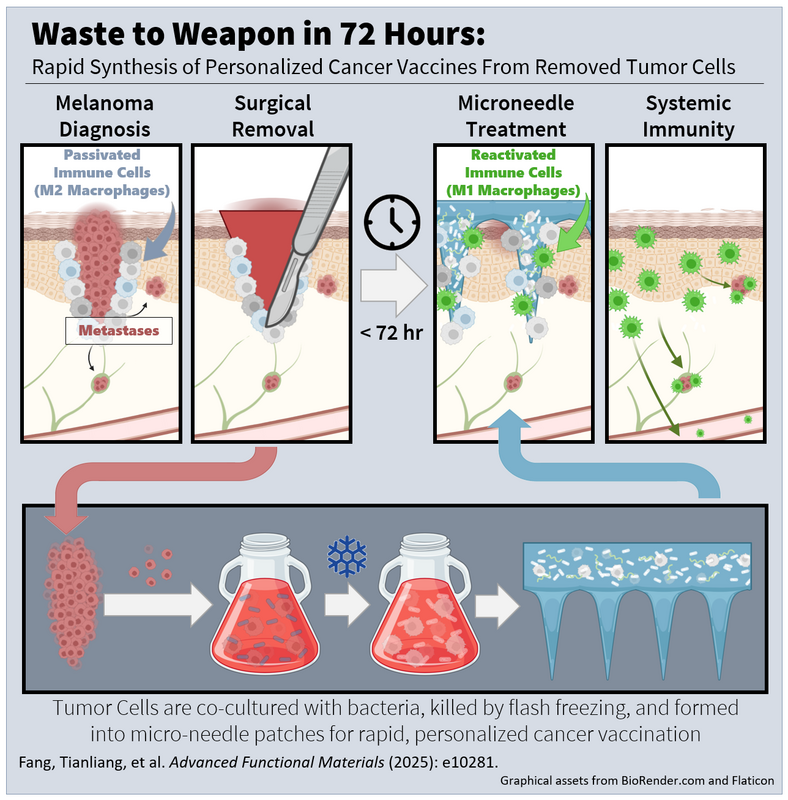
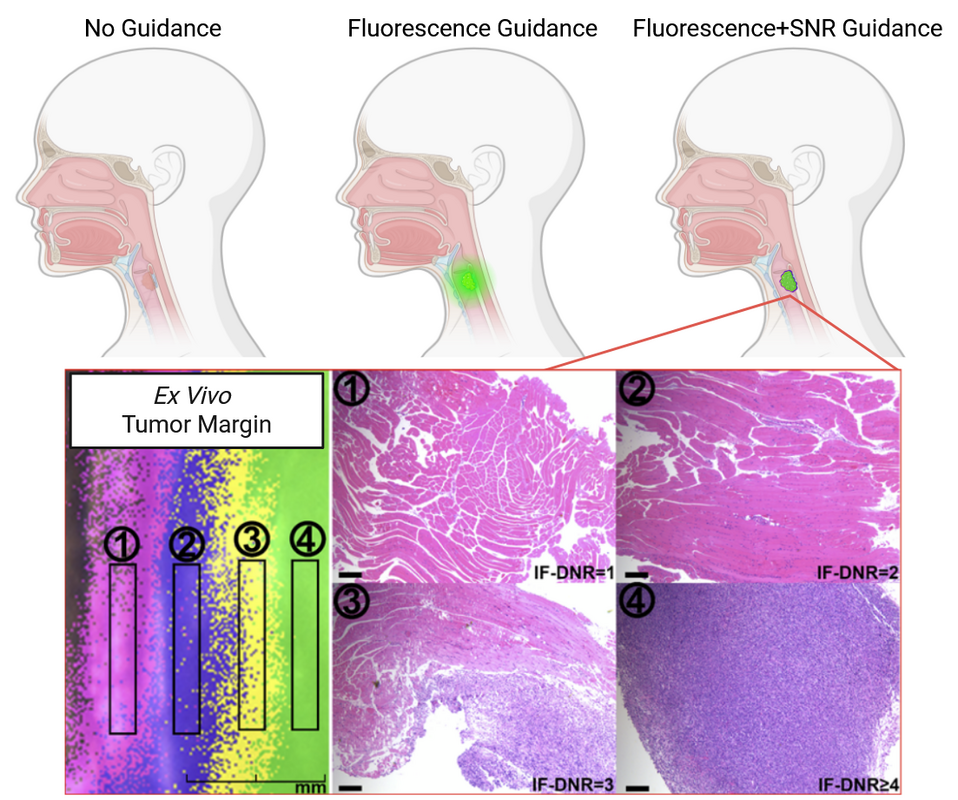
- Published on
We've published an important review led by Prof Sheng of Shenzhen Institute for Advanced Technology in the Journal of Nanobiotechnology, which Professor Wang and I are happy to have contributed to. The review systematically examines cell membrane-engineered nanoparticles for brain-targeted drug delivery, providing guidance for future research in this challenging field.
The blood-brain barrier remains one of the most significant obstacles in treating neurological diseases, blocking over 98% of potential therapeutics. While various nanocarrier approaches have emerged, the field lacks a systematic comparison of different cell membrane sources and how different membrane sources cross this barrier.
We systematically compared six membrane types—red blood cells, platelets, tumor cells, macrophages, neutrophils, and NK cells—each exploiting distinct transport mechanisms. Red blood cell membranes provide immune evasion, tumor cell membranes enable disease-specific targeting, and immune cell membranes leverage natural inflammatory pathways. These work through receptor-mediated transport, tight junction modulation, or direct membrane penetration, with each approach offering complementary strengths and limitations.
Our analysis reveals significant performance variations across membrane types. Red blood cell carriers show excellent circulation times but require additional targeting modifications, while tumor cell membranes provide inherent disease specificity but face scalability challenges. Immune cell membranes offer natural inflammatory targeting but have shorter circulation half-lives. Hybrid membrane approaches attempt to combine advantages but remain understudied.
What makes this review especially valuable is the identification of critical translation barriers and future research directions. We outline key challenges including standardized manufacturing protocols, comprehensive safety characterization, and the need for mechanistic validation studies. The integration of artificial intelligence for rational design and the development of personalized membrane sources represent promising avenues for advancement.
We believe this systematic analysis provides an important roadmap for researchers working to overcome blood-brain barrier delivery challenges and advance these promising biomimetic platforms toward clinical translation.
The blood-brain barrier remains one of the most significant obstacles in treating neurological diseases, blocking over 98% of potential therapeutics. While various nanocarrier approaches have emerged, the field lacks a systematic comparison of different cell membrane sources and how different membrane sources cross this barrier.
We systematically compared six membrane types—red blood cells, platelets, tumor cells, macrophages, neutrophils, and NK cells—each exploiting distinct transport mechanisms. Red blood cell membranes provide immune evasion, tumor cell membranes enable disease-specific targeting, and immune cell membranes leverage natural inflammatory pathways. These work through receptor-mediated transport, tight junction modulation, or direct membrane penetration, with each approach offering complementary strengths and limitations.
Our analysis reveals significant performance variations across membrane types. Red blood cell carriers show excellent circulation times but require additional targeting modifications, while tumor cell membranes provide inherent disease specificity but face scalability challenges. Immune cell membranes offer natural inflammatory targeting but have shorter circulation half-lives. Hybrid membrane approaches attempt to combine advantages but remain understudied.
What makes this review especially valuable is the identification of critical translation barriers and future research directions. We outline key challenges including standardized manufacturing protocols, comprehensive safety characterization, and the need for mechanistic validation studies. The integration of artificial intelligence for rational design and the development of personalized membrane sources represent promising avenues for advancement.
We believe this systematic analysis provides an important roadmap for researchers working to overcome blood-brain barrier delivery challenges and advance these promising biomimetic platforms toward clinical translation.